Mega Doctor News
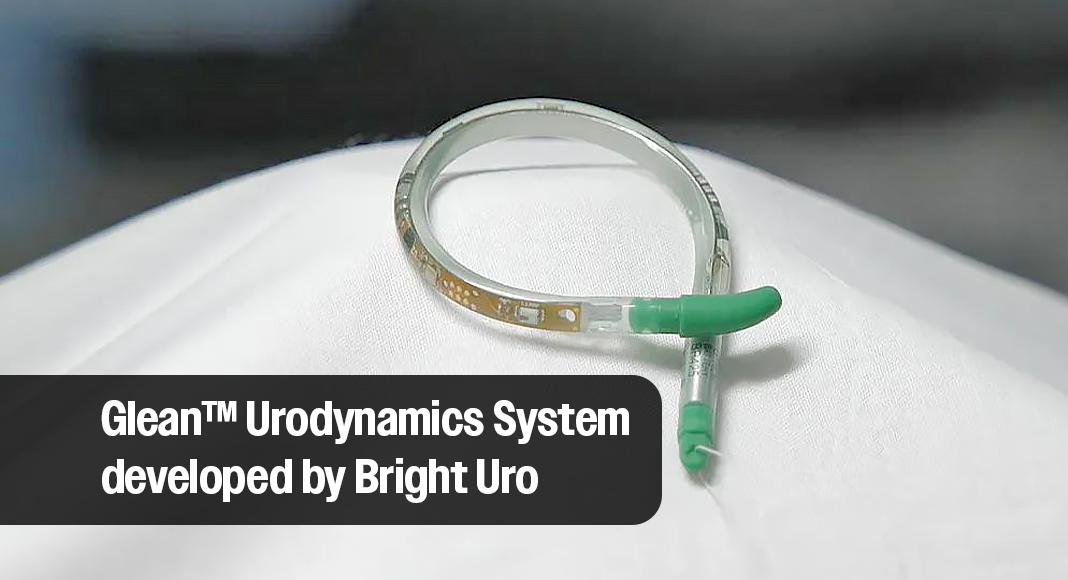
CLEVELAND CLINIC – Cleveland Clinic is the first to use the Glean™ Urodynamics System, the first wireless, catheter-free method of urodynamic monitoring following 510(k) clearance from the U.S. Food and Drug Administration in March.
Leveraging foundational technology generated from research conducted by Cleveland Clinic, Bright Uro created the Glean Urodynamics System which offers more comfortable, accurate testing and the ability to monitor bladder activity during normal daily activities.
Cleveland Clinic identified this critical unmet need in response to conventional urodynamic assessments (tests that demonstrate how well the bladder, sphincters, and urethra hold and release urine to determine the source of leaks or blockages), which have several limitations.
The FDA clearance is a prime example of a clinical innovation advancing from inception to reality and now being offered to patients, as researchers in Cleveland Clinic’s Department of Urology and Lerner Research Institute in collaboration with researchers at Louis Stokes Cleveland VAMC and Case Western Reserve University invested more than a decade developing the original technology.
“After observing standard urodynamic testing over the past 20 years, our vision for this device was to address the shortcomings in current bladder testing,” said Margot Damaser, Ph.D., leader of the research team and a staff member in the Biomedical Engineering Department of Cleveland Clinic’s Lerner Research Institute. “We wanted to invent a novel solution that would take the monitoring out of an artificial setting and eliminate the need for a catheter, which can be painful and embarrassing.”
Watch the Cleveland Clinic Video Below:
Published data in The Journal of Urology in July 2023 confirmed the device’s safety, while demonstrating that it did not impede lower urinary tract function and could reliably identify bladder events when compared to conventional urodynamics.
An estimated 80 million Americans suffer from voiding dysfunction, including overactive bladder, urinary incontinence, enlarged prostate and neurogenic bladder. Catheter-based testing can be painful and embarrassing because patients must force urination in an office setting to replicate the symptoms they experience in their daily lives. Because of the artificial conditions and a catheter obstructing the urethra, the simulation is not always a physiologic reflection of patients’ experiences and often generates inaccurate data.
Glean offers streamlined testing that is easier for clinicians to use, delivers more accurate data and offers a more comfortable experience for the patient.
The Glean bladder sensor consists of a silicone tube that houses the pressure-sensing technology and curls into a coil shape upon insertion to remain within the bladder. The device may utilize natural physiology to fill the bladder while gathering diagnostic data. In 2022, Bright Uro secured a license to the original technology generated by the Cleveland Clinic.
Dr. Damaser led the initial clinical research along with Dr. Howard Goldman, a urologist in the Glickman Urological Institute, in addition to researchers and physician collaborators at Case Western Reserve University and the Louis Stokes Cleveland VA Medical Center.
Dr. Goldman was the first physician to use the device in a clinical setting. “By enabling monitoring of the bladder without a catheter in place, this technology can offer a more accurate and patient-centered approach to diagnosing lower urinary tract dysfunction, which can also increase treatment rates,” said Dr. Goldman.
“We are so excited to bring this important innovation into clinical practice where it will help clinicians and patients across the US,” said Derek Herrera, CEO of Bright Uro. “The level of enthusiasm is truly remarkable, as we hear from so many clinicians who believe Glean will transform how we diagnose bladder dysfunction. It’s especially meaningful that the first commercial case is taking place at Cleveland Clinic, where this concept originated. We’re deeply grateful to all the researchers and clinical teams who worked tirelessly to lay the foundation for us to transform the field.”











